Carbon dioxide is one of the earliest “natural” products. Although carbon dioxide currently only makes up about 0.03% of the atmosphere it is possible that, in the early stages of the development of the earth, it could have consisted of as much as 80% of Earth’s atmosphere. These high concentrations of carbon dioxide were produced by the volcanic activity which was ubiquitous across the Earth at that time. Indeed, most “natural” carbon dioxide sources which exist on the Earth today are produced from magma deep underneath the Earth’s surface. Over the course of billions of years the high concentrations of atmospheric carbon dioxide were reduced when sea-dwelling life and, eventually, plankton, plants and trees, evolved the process of photosynthesis. This process released oxygen and sequestered carbon in the form of carbonate minerals, oil shale, coal, and petroleum within Earth’s crust. Since the start of the industrial revolution the consumption of these materials by a range of “human“ activities has re-released carbon dioxide into the atmosphere.
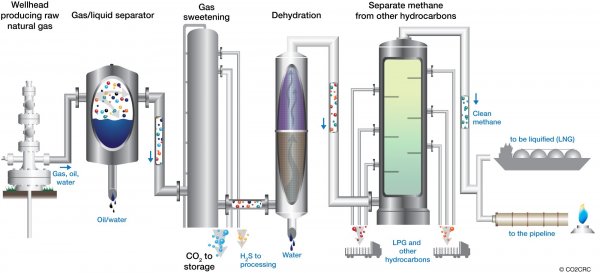
Although the combustion of carbonaceous materials generates a large amount of carbon dioxide this material is usually only present in the gaseous products of such reactions at concentrations of about 10%. This concentration is considered to be too low to allow the economic recovery of carbon dioxide from such streams and these types of emissions are not commonly used as sources of carbon dioxide. Natural gas streams may also contain significant concentrations of carbon dioxide (up to about 20%) and this carbon dioxide is often separated in order to upgrade the quality of the methane rich product gas. However, most of this carbon dioxide is simply vented or, in some cases, re-injected into the well. Hence, at the moment, the carbon dioxide which is used in downstream processing applications is only recovered from sources in which the carbon dioxide concentration within the raw gas is very high. The principal sources of carbon dioxide which are used in commercial processes are listed below. These sources can be categorized as those associated with natural processes, those resulting from fermentation processes and those produced by chemical processing.
CO2 Might Produced form Many Sources :
Natural Sources
These sources of carbon dioxide are produced by the Earth’s natural volcanic activity and can include both geothermal sources and natural wells. Carbon dioxide can be found in underground wells at concentrations of 90% to almost 100% depending on the location of the well. Large carbon dioxide wells exist in the United States (e.g. in Colorado, Mississippi, New Mexico, Utah and Wyoming) and in Europe (e.g. at Répcelak and Oelboe in Hungary and at Bad Driburg-Herste and Rottenburg in Germany). Geothermal carbon dioxide is found in numerous locations across Spain and Italy (e.g. at Torre Alfina).
Fermentation
The fermentation of sugars and starches (catalyzed by yeast) produces ethanol and gaseous carbon dioxide. This reaction is commonly employed where the use of agricultural-based ethanol as a fuel additive is actively encouraged (e.g. the federal tax system in the U.S. exempts agricultural-based ethanol-gasoline blends from motor fuel excise tax) . The reaction is also the basis for brewing operations around the world. The carbon dioxide produced by fermentation processes can be of high purity but can be tainted by odors.
Chemical Processing
Hydrogen or Ammonia Synthesis
Large quantities of high quality, by-product, carbon dioxide are produced by plants which are dedicated to the manufacture of hydrogen or ammonia. These plants operate by steam reforming natural gas, LPG or naphtha into a mixture of synthesis gas (i.e. hydrogen , carbon monoxide and carbon dioxide) and the carbon monoxide is then catalytically removed by forming additional carbon dioxide via the water-gas shift reaction. The carbon dioxide “by-product” is then removed from the desired hydrogen product by using an amine based absorption system or, more commonly, by a PSA system. The carbon dioxide which is produced by this process tends to be free from many troublesome impurities since these are removed upstream of the steam reforming process in order to protect the highly dispersed metal catalysts which are employed in these units.
Ethylene Oxide
The controlled oxidation of ethylene can produce ethylene oxide. However, the selectivity for this reaction over commercially available silver-based catalysts is only about 80% and the non-selective, total, oxidation of ethylene can occur to produce carbon dioxide.
Acid Neutralization
Calcium carbonate (which is commonly known as limestone) is used in many locations to neutralize the acids which exist within waste streams from industrial processing. This simple, acid-base reaction results in the production of good quality, gaseous, carbon dioxide.
Other Industrial Sources
A variety of other chemical operations have been used to produce carbon dioxide. For example, the processing of phosphate rocks can release carbon dioxide. The impurity profile of this source of carbon dioxide can be unusual since it may contain traces of phosphines or radon gas. Combustion processes are used in generators to produce energy by the reaction of a hydrocarbon with oxygen. This reaction results in the production of a by-product flue gas stream consisting of nitrogen and carbon dioxide. Since the hydrocarbon fuel is not commonly purified prior to combustion the carbon dioxide product of this process can contain an unusual range of impurities (e.g. mercury, organochlorides and HCN).

